Pharma trends for 2026 and beyond

January 5, 2026 9 min read
We’re entering a phase where pharma’s biggest shifts aren’t theoretical anymore — they’re already reshaping daily work. Analysts at McKinsey and ZS agree: AI is moving out of pilot mode and into full‑scale programs across drug discovery, clinical trials, supply chain, and commercial operations. The numbers speak for themselves: AI in pharma is projected to grow from roughly US $1.9 billion in 2025 to more than US $16 billion by 2034.
The combination of generative AI and “agentic” AI — systems that can chain reasoning, interact with tools, and take actions — is expected to generate tens of billions annually across the full pharmaceutical value chain, transforming AI from a smart assistant into an active workflow partner.
But there’s a caution flag — unless projects are anchored in clear business value and governed carefully, over 40% of agentic AI initiatives are expected to be cancelled by 2027.
This article is your roadmap for 2026 and beyond: how the tools matured, which bets are paying off, where regional dynamics are shifting — and what you can do this quarter to test and scale what truly works.
AI moves from insight to action
Generative AI is no longer limited to content or documentation. According to McKinsey, it could generate up to $60‑110 billion/year in value across discovery, clinical, operations, and commercial functions.
Meanwhile, the market for AI‑driven drug discovery alone is forecast to reach about US $14 billion by 2030 (from under $1 billion just a few years ago) — reflecting rapid adoption of computational screening, predictive modeling, and molecular optimization.
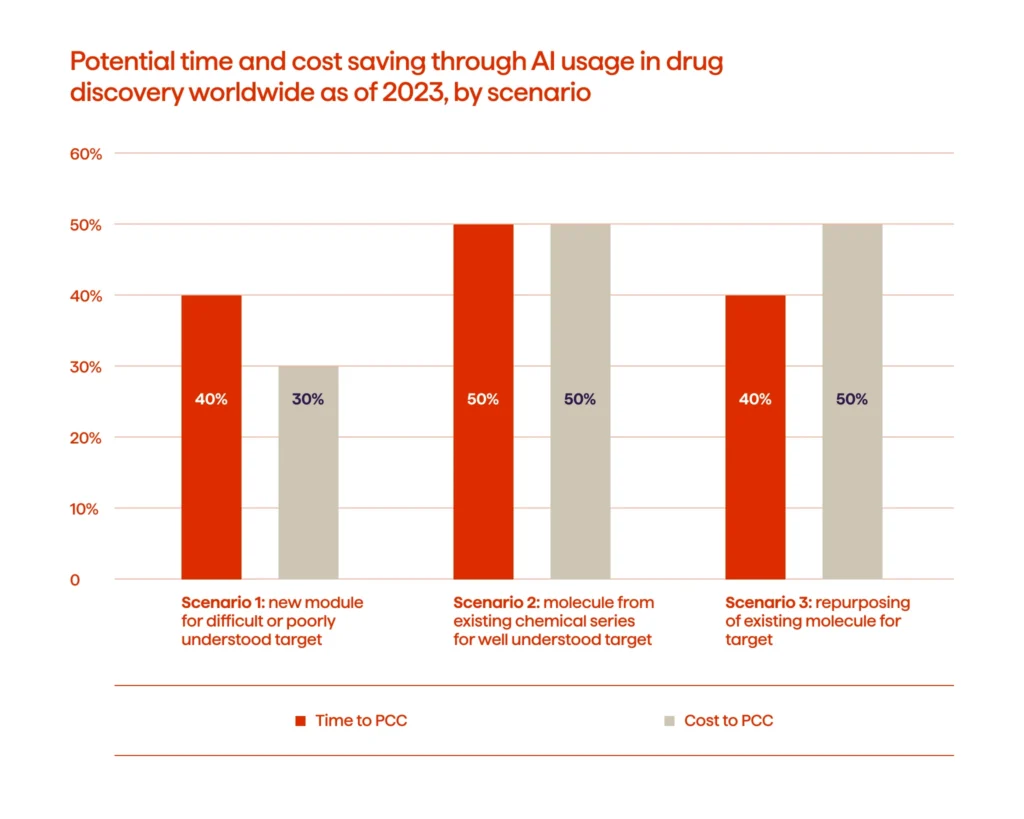
Recent evidence also shows that AI-enabled discovery workflows are delivering measurable operational gains. For complex targets, these approaches can shorten early discovery timelines by up to 40% and reduce costs by roughly 30%, giving teams room to test more ideas without extending project cycles.
This shift is also visible in new partnerships and data‑sharing consortia among established pharma players aiming to pool data and accelerate discovery — a sign that AI-powered workflows are becoming strategic rather than experimental.
What this means for you: Firms ready to integrate AI across discovery, R&D, and commercialization are positioning themselves for scale, but such integration depends critically on data infrastructure, cross‑functional alignment, and clear ROI metrics.
Cloud-native data platforms and digital cores
Cloud and edge computing are now central to scalable R&D, enabling real-time collaboration and AI integration across global teams. By adopting cloud-first architectures, organizations can unify multi‑omics, imaging, clinical, and real-world data — cutting infrastructure costs and speeding AI-driven workflows.
The numbers speak for themselves: 42% of pharma firms use cloud-based platforms, driving 52% faster trial timelines and 48% improved data integration efficiency. Overall, AI in pharma and biotech is projected to grow from $2.35 B in 2025 to $7.61 B by 2034 (≈14% CAGR), much of it fueled by cloud-ready systems.
Your action point: If your infrastructure still relies on fragmented, siloed systems, prioritizing a unified, cloud-based data core is one of the most important moves you can make this quarter.
Software as the backbone of next-generation MedTech
The MedTech in 2026 analysis points to a clear shift: medical devices are increasingly defined by the software running inside them. The embedded software market alone is projected to grow from $17.9B in 2024 to about $30.2B by 2030 (≈9.5% CAGR), driven by rising investment in the code that powers surgical robots, wearables, and connected monitoring tools.
This shift has practical consequences. Devices are now expected to collect, process, and transmit data continuously — supporting chronic disease management, mental health monitoring, and next-generation diagnostics. As IoMT becomes a baseline capability, expectations around reliability, interoperability, and real-world performance are getting higher.
To keep pace, testing has become a non-negotiable part of the product lifecycle. Automated and simulation-based frameworks now validate everything from firmware behavior to connectivity and cloud integrations. These layers of verification are increasingly what determine whether a device is clinically trustworthy, especially as more tools move into home environments where consistency and safety can’t be compromised.
Tip: When you assess new product lines or plan upgrades, focus on devices with strong embedded software foundations and predictable data pipelines. Reliable integration across sensors, firmware, connectivity, and cloud services will shape real-world performance — and directly influence clinical adoption.
AI-driven clinical development and the continued rise of digital trials
Clinical development is moving toward faster, more distributed, and data-heavy models. Trial starts have stabilized at pre-pandemic levels, R&D investment is rising again, and productivity is improving — yet enrollment still slows down most programs. AI is beginning to close that gap. Recent analyses show AI-assisted protocol design and analytics can reduce trial duration by about 10%, lower operational costs, and improve success probability.
Digital recruitment tools built on EHR and claims data are also gaining traction, helping teams find eligible participants more efficiently and reach more diverse groups. This shift is reinforced by decentralization: hybrid and virtual models supported by wearables, remote assessments, and telemedicine are now standard elements of trial design. Real-world data and digital endpoints now appear in a growing share of studies, giving teams earlier insight into adherence, safety, and day-to-day patient behavior.
Geographic patterns are changing too. Western Europe’s share of global trial activity fell from 32% in 2019 to 28% in 2024, and Central/Eastern Europe dropped from 16% to 11%, a sign that activity is spreading across emerging regions with broader patient access.
A practical next step: Bringing AI-supported recruitment, digital endpoints, and remote monitoring into your plans early can help you secure more predictable timelines and stronger evidence packages.
When diagnostics get smarter, care gets more personal
AI is changing the way teams detect and understand disease, especially in areas where early signals are easy to miss. Trend reports for 2026 point to steady growth in AI imaging, automated pathology, and predictive models that help clinicians make clearer, faster decisions. These tools are becoming a practical part of moving toward more individualized care.
You can already see the impact in day-to-day practice. In the NHS, an AI review of existing CT scans flagged previously undiagnosed vertebral fractures in more than 2,000 patients. That matters, given that osteoporosis leads to fractures in half of all women and one in five men over 50, and each hip fracture costs the system more than £16,000 on average. Catching issues earlier gives clinicians more room to intervene before they become far more serious.
Across BioTech, the same shift is taking shape. AI-supported diagnostics are being paired with cloud analytics and real-time data processing to build a fuller picture of patient risk and likely outcomes. As these models mature, they’re helping teams identify the right patients earlier, tailor treatments more confidently, and cut down on guesswork during clinical development.
Something worth considering: Bringing AI-enabled diagnostics into your research or clinical work can give you earlier signals and more precise guidance, helping you shape stronger treatment strategies from the start.
Customer engagement enters an agentic phase
Customer engagement in pharma is set for a noticeable shift in 2026 as agentic AI moves from internal workflows into real customer-facing use. Early rollouts of platforms like Agentforce Life Sciences for Customer Engagement point to a future where interactions with HCPs, partners, and patients are no longer driven by static CRM journeys, but shaped dynamically by context, timing, and intent.
Instead of preparing everything in advance, teams gain support in the moment. Agentic systems can coordinate next steps during interactions, surface relevant insights as conversations unfold, and reduce the manual follow-ups that slow commercial and medical teams down. That changes how relationships are managed day to day — and how quickly organizations can respond across markets.
What sets this shift apart is that these systems don’t stop at insight. Within defined guardrails, they can help carry actions forward, while keeping humans firmly in control. This is also where focused investment and practical rollout experience matter. At Avenga, this is one of the areas we’re actively building toward — not as a standalone capability, but as part of a broader push to connect data, AI, and engagement in ways that hold up in real operating environments.
Why this matters now: As agentic engagement platforms start entering production, the teams that will benefit most are those aligning commercial, medical, and compliance early — with clean data, clear vision, and realistic expectations about where autonomy adds value and where human judgment still leads.
Keeping agentic AI within safe boundaries
Agentic AI can take on more steps in a workflow, but this also creates room for things to go wrong if the system isn’t tightly governed. Microsoft’s recent approach in Windows 11 makes that clear: cross-prompt injection can push an agent to run actions users never intended or move data without proper visibility. That’s why they now require admin-level activation, separate agent identities, and tamper-proof audit trails.
In pharma and life sciences, the impact is even more serious. CIOMS points out that when AI systems operate across safety, regulatory, or clinical tasks, the risks go beyond model accuracy — they touch decision accountability, data access, and compliance.
Recommendation: Build in permission controls, auditability, and human approval points early in the design. These guardrails are far more effective when they’re part of the core architecture, not patches added after deployment.
Key steps for this quarter
- Audit your data platforms — ensure cloud-native, compliant, and connected systems.
- Select 1–2 high-value AI use cases — such as molecule generation, trial recruitment, or supply chain optimization.
- Plan decentralized trials — integrate wearables, telemedicine, and real-world data.
- Embed software-first MedTech — make devices part of your AI ecosystem.
- Govern rigorously — establish audit logs, human-in-the-loop controls, and compliance protocols.
- Measure impact — track metrics such as time-to-candidate, cost per patient enrolled, and compliance incidents.
Looking ahead
2026 isn’t about debating whether AI has potential in pharma — it’s about integrating it into how the industry works. Generative and agentic AI, cloud data platforms, decentralized trials, and software‑first MedTech devices are all converging. Organizations that build their infrastructure, governance, and workflows now will turn potential into performance.
If you have questions or want to explore how these AI advancements can be applied to your specific context, feel free to reach out.