The Power Of A Pharmacy Database Management System

May 6, 2025 11 min read
Discover how this powerful tool optimizes operations, enhances efficiency, and helps ensure regulatory compliance.
The pharmaceutical industry thrives on innovation and efficiency. To stay ahead in this competitive landscape, organizations need to be able to process, manage, and extract insights from large, comprehensive sets of data. Enter the pharmaceutical database management system: a tool that streamlines information-related workflows, fueling R&D, and ultimately propelling business growth.
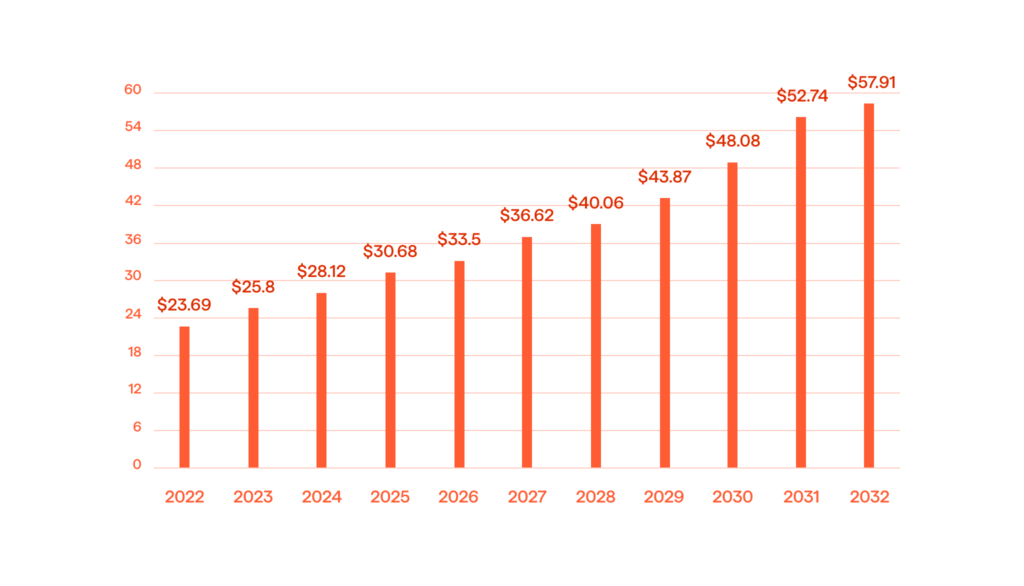
The global pharmacy management system market size is expected to reach USD 57.91 billion by 2032.
This article delves into the key advantages of utilizing a pharma database management system, highlighting how it empowers pharma companies, large and small, to make informed decisions, optimize processes, and gain a competitive edge.
What is a pharmaceutical database management system?
A pharma database management system [PDMS] is a software application designed to handle the specific data needs of the pharmaceutical industry. It goes beyond a general database by offering features and functionalities relevant to drug research, clinical trials, inventory management, and even pharmacy operations.
Here’s a breakdown of what a pharma PDMS typically offers:
- Data storage and management. Stores and organizes vast amounts of complex data, including drug information, chemical structures, clinical trial results, and patient records.
- Regulatory compliance. Helps ensure data adheres to strict regulations governing drug development and pharma record-keeping.
- Inventory management. Enables easier tracking of drug inventory levels, expiry dates, and ordering processes for pharmacies and research facilities.
- Reporting and analytics. Could have built-in reporting features, meaning it can generate reports and analysis of data relevant to research, and track drug development progress.
- Security and access control. Provides robust security measures to protect sensitive patient and drug information.
So, in essence, a pharma PDMS helps streamline data management across the entire pharmaceutical lifecycle, from research and development to manufacturing and distribution.
PDMS Benefits: Detailed Breakdown
A PDMS can be highly beneficial in various aspects of the pharmaceutical industry. Here are some key advantages.
Centralized Data Storage
Scattered data hinders progress in pharma. A PDMS centralizes everything for better analysis, security, and collaboration.
- Data consolidation. The pharmaceutical industry deals with vast amounts of data from various sources, such as clinical trials, research studies, manufacturing processes, and regulatory submissions. Without a centralized system, this data is often scattered across different systems, departments, and locations, making it challenging to access, share, and analyze. A PDMS provides a single, centralized repository where all relevant data can be stored and managed.
- Data integrity and consistency. With data stored in multiple locations, there is a higher risk of inconsistencies, redundancies, and errors. A centralized PDMS ensures that all data is stored in a consistent format, following standardized naming conventions and data structures. This enhances data integrity, reduces redundancies, and minimizes the chances of errors or conflicting information.
- Data security and access control. Pharmaceutical data often contains sensitive and confidential information, such as patient data, proprietary formulations, and trade secrets. A centralized PDMS can implement robust security measures, including access controls, user authentication, and data encryption, to protect this valuable data from unauthorized access or breaches.
- Efficient data retrieval and analysis. With data stored in a centralized location, it becomes easier and faster to retrieve and analyze data from a single source. This facilitates more efficient data mining, reporting, and decision-making processes, as users don’t have to navigate multiple systems or locations to gather the required information.
- Collaboration and information sharing. A centralized PDMS enables seamless collaboration and information sharing among different teams, departments, and stakeholders involved in the drug development and manufacturing processes. This promotes cross-functional collaboration, facilitates knowledge transfer, and enhances overall productivity.
- Regulatory compliance. Regulatory agencies often require pharmaceutical companies to maintain comprehensive data records and provide evidence of data integrity, traceability, and audit trails. A centralized PDMS can help companies comply with these regulatory requirements by maintaining a secure, auditable, and easily accessible data repository.
- Scalability and flexibility. As pharmaceutical companies grow and their data needs evolve, a centralized PDMS can be scaled and adapted to accommodate increasing data volumes and new data types, without the need for significant changes to existing systems or processes.
A pharmaceutical database management system streamlines data management, improves data quality and accessibility, and provides a solid foundation for efficient drug development, manufacturing, and regulatory compliance processes by consolidating data into a centralized repository.
Enabling Compliance
The pharmaceutical industry is subject to heavy regulatory scrutiny, and a PDMS usually plays a vital role in supporting compliance efforts. Regulatory compliance is needed for several reasons.
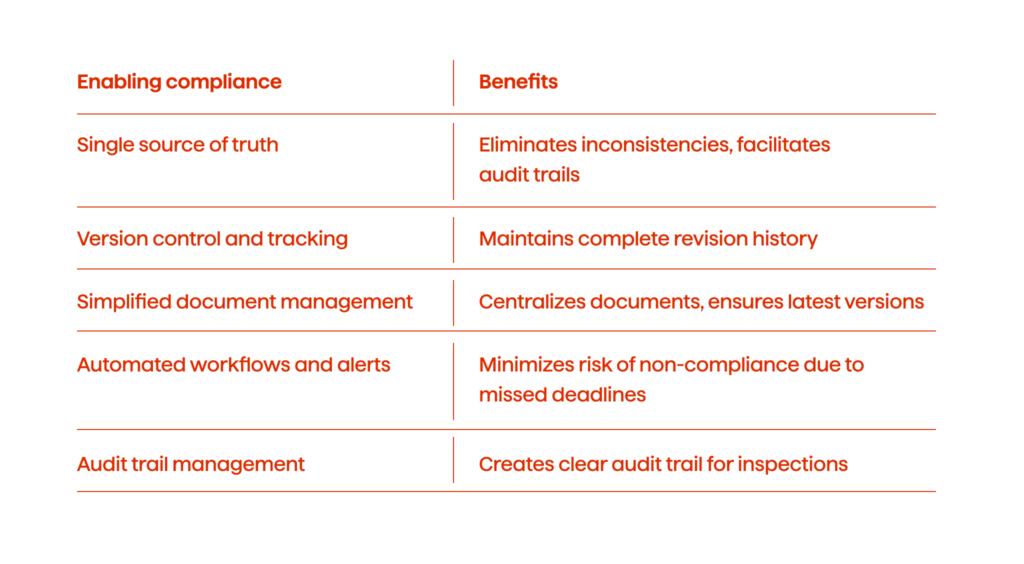
- Single source of truth. PDMS acts as a single source of truth for all product information, from formulation details to manufacturing processes. This eliminates inconsistencies and facilitates easy access to audit trails, a key requirement for regulatory bodies.
- Version control and tracking. In PDMS, the changes made to product data, can be meticulously monitored, wich allows companies to maintain a complete history of revisions. This ensures compliance with regulations demanding detailed documentation of any modifications.
- Simplified document management. PDMS eliminates the need for paper-based documentation, centralizing Standard Operating Procedures (SOPs), batch records, and other regulatory documents. This simplifies document retrieval and ensures all parties are working with the latest approved versions.
- Automated workflows and alerts. PDMS can automate tasks like expiration notifications for critical documents or trigger alerts for upcoming regulatory deadlines. This proactive approach minimizes the risk of non-compliance due to missed deadlines.
- Audit trail management. PDMS maintains a detailed record of all actions taken within the system, creating a clear audit trail for regulatory inspections. This transparency demonstrates a commitment to compliance and simplifies the audit process.
By implementing a PDMS that supports regulatory compliance, pharmaceutical companies can streamline their operations, enhance data integrity and traceability, facilitate regulatory submissions, and ultimately contribute to the development and delivery of safe and effective drug products while minimizing regulatory risks and liabilities.
Inventory Management
Effective inventory management is essential for ensuring the availability of drugs, minimizing waste, and maintaining compliance with regulatory requirements. Here’s how a PDMS can help organizations manage inventory more efficiently.
- Centralized inventory data. The PDMS serves as a centralized repository for storing and managing inventory data, including information on drug products, raw materials, packaging materials, and other supplies. It consolidates inventory data from various sources, such as manufacturing facilities, distribution centers, and warehouses, providing a comprehensive view of inventory levels across the supply chain.
- Inventory tracking and monitoring. The PDMS can track and monitor inventory levels in real time, enabling accurate visibility into stock levels, expiration dates, and lot/batch numbers. It can generate alerts and notifications when inventory levels fall below predefined thresholds, allowing for timely reordering and replenishment.
- Inventory forecasting and planning. PDMS systems often include forecasting and planning tools that analyze historical demand patterns, production schedules, and other relevant data to predict future inventory requirements. This information can be used to optimize inventory levels, reduce excess stock, and ensure sufficient supply to meet demand.
- Lot/batch traceability. The PDMS maintains detailed records of lot/batch information, including manufacturing dates, expiration dates, and quality control data. This traceability is crucial for managing product recalls, investigating quality issues, and complying with regulatory requirements for product traceability.
- Integration with manufacturing and distribution systems. The PDMS can integrate with other systems used in pharmaceutical manufacturing and distribution, such as Enterprise Resource Planning (ERP) systems, Manufacturing Execution Systems (MES), and Warehouse Management Systems (WMS). This integration enables seamless data exchange and coordination between inventory management and other operational processes.
- Cost optimization. By optimizing inventory levels and minimizing waste, a PDMS can help pharmaceutical companies reduce carrying costs, storage costs, and the costs associated with expired or obsolete stock.
Effective inventory management through a PDMS ensures the availability of drugs, minimizes stockouts and shortages, reduces waste, and supports regulatory compliance, ultimately contributing to the efficient and cost-effective distribution of pharmaceutical products.
Learn more about how we automated budget processes and enhanced the operational efficiency of a leading biopharma enterprise. Success story
Elevating Collaboration
An advanced PDMS facilitates collaboration and information sharing within the pharmaceutical industry. Collaboration and information sharing are essential for various reasons:
- Cross-functional teamwork. Drug development and manufacturing processes involve multiple teams and departments, such as research and development, clinical operations, regulatory affairs, quality assurance, and manufacturing. A PDMS provides a centralized platform for these teams to access, share, and collaborate on relevant data, enabling effective communication and coordination across functions.
- Global collaboration. Pharmaceutical companies often operate globally, with teams and facilities spread across different locations and countries. A PDMS enables remote access to shared data repositories, allowing geographically dispersed teams to collaborate seamlessly and work on the same set of data.
- External partnerships. Pharmaceutical companies frequently collaborate with external partners, such as academic institutions, contract research organizations (CROs), and other industry partners. A PDMS can provide controlled access and secure data-sharing capabilities, facilitating collaboration while maintaining data privacy and confidentiality.
An advanced PDMS acts as a central hub for collaboration and information exchange across teams, departments, and even external partners in the pharmaceutical industry.
Choosing Between A Ready-made And Custom Pharma PDMS
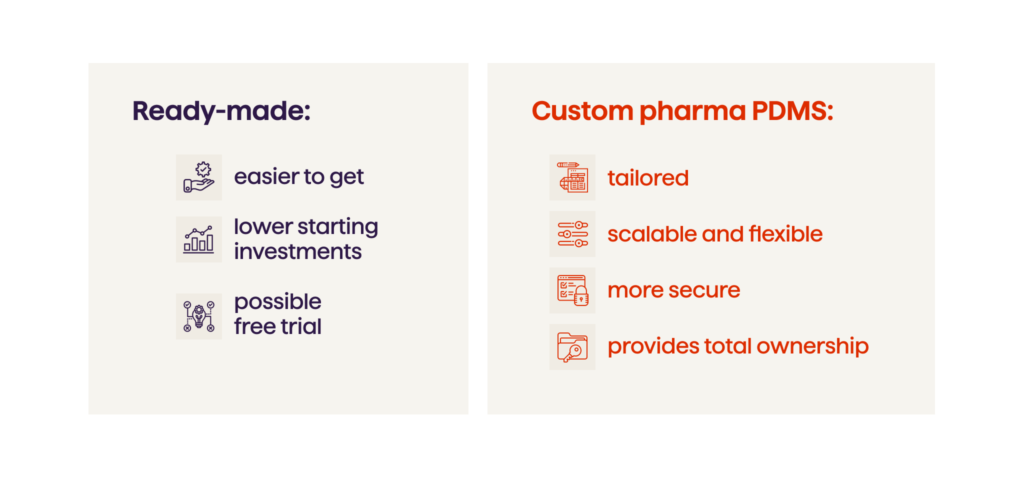
The choice between developing a custom PDMS or purchasing a ready-made one requires careful consideration. Off-the-shelf solutions offer convenience of the immediate deployment, but you can’t really expect them to be efficient at addressing the unique intricacies and evolving needs of a specific pharma organization. On the other hand, building a sophisticated application from scratch is far more substantial an endeavor, requiring more commitment and recourses. However, this approach is vastly more advantageous in the long run. Here are the benefits of a custom solution.
Tailored Precision
Ready-made tools are designed to cater to a broad spectrum of industries, resulting in a one-size-fits-all approach that may not perfectly align with the workflows and operational nuances of pharma organizations. Conversely, a custom-built system is crafted with precision to seamlessly integrate into your existing infrastructure and workflows. From inventory management to patient records and regulatory compliance, every facet of your pharmacy’s operations can be meticulously tailored to optimize efficiency and accuracy.
Scalability And Flexibility
Pharmacy operations are dynamic, subject to evolving regulations, technological advancements, and fluctuating demand. While a ready-made PDMS may suffice initially, it may quickly become obsolete or inadequate as your pharmacy grows or adapts to industry changes. With a custom solution, scalability and flexibility are inherent features. Your system can be designed to accommodate future expansions, incorporate new functionalities, and adapt to regulatory updates without the constraints imposed by pre-packaged software limitations.
Enhanced Security And Compliance
As we’ve already stressed above, the confidentiality and integrity of patient data are paramount in the pharmaceutical domain, necessitating robust security measures and compliance protocols. Off-the-shelf solutions may offer standard security features, but they often lack the depth of customization required to meet the stringent regulatory requirements specific to your geography. By opting for a custom-built PDMS, you gain the ability to implement tailored security protocols, encryption standards, and access controls tailored to your unique needs, ensuring compliance with HIPAA, GDPR, or any other relevant regulations.
Total Ownership And Control
When you invest in a custom pharmacy PDMS, you retain full ownership and control over your system. Unlike off-the-shelf solutions, where you are at the mercy of third-party vendors for updates, support, and feature enhancements, a custom solution empowers you to dictate the roadmap of your system’s development. Whether it’s adding new functionalities, integrating with external systems, or optimizing performance, the ability to customize and evolve your PDMS according to your pharmacy’s evolving needs provides unparalleled autonomy and long-term cost-effectiveness.
A pharmaceutical PDMS offers various benefits to organizations both in terms of operational efficiency and security, but only if its selected and implemented properly. Pharma companies can sometimes opt for ready-made solutions, some of which are quite rich in functionality, especially if they’re limited in time and resources. However, only a custom data management tool can be tailored completely to unique needs and workflows.
To Sum Up
The pharmaceutical landscape is as fiercely competitive as ever, and developing a custom system could be a powerful strategic move, allowing organizations to outperform rivals. This type of pharmaceutical PDMS can be truly transformative. It can enable businesses to gain enhanced operational excellence, scalability, and security and even help innovate faster.
If you want to unlock the power of pharmaceutical database management systems or build a custom solution to optimize your organization’s performance – contact Avenga right now for a free consultation.