The Potential Of Digital Twins In Biopharmaceutical Manufacturing

May 7, 2025 13 min read
The introduction of digital twin technology into the biopharmaceutical sector holds promise for improved manufacturing processes.
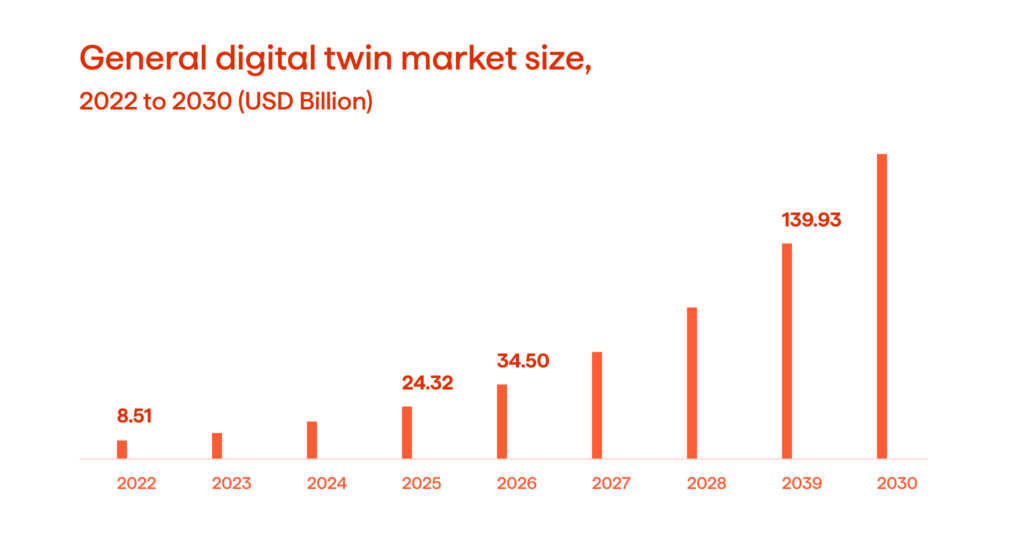
The concept of digital twins (DT) is well-established in many industries. It’s enabled by Industry 4.0 technologies that aim to create smart and connected production systems that can sense, predict, and interact with the physical world to support production in real-time.
The pharmaceutical sector is going through a digital transformation to embrace Industry 4.0. According to Deloitte, the biopharmaceutical production process is currently at a turning point in digital innovation, but DTs haven’t been extensively adopted yet. In this article, we’ll discuss the use of digital twins in the production of biopharmaceuticals, along with the advantages and difficulties of this technology and its prospects.
Understanding Digital Twins Technology
A digital twin is an advanced simulation model that bridges the physical and digital worlds within the manufacturing environment. It comprises three key elements: a physical component, a virtual component, and data communication. The seamless interaction between these elements is facilitated by an integrated data management system.
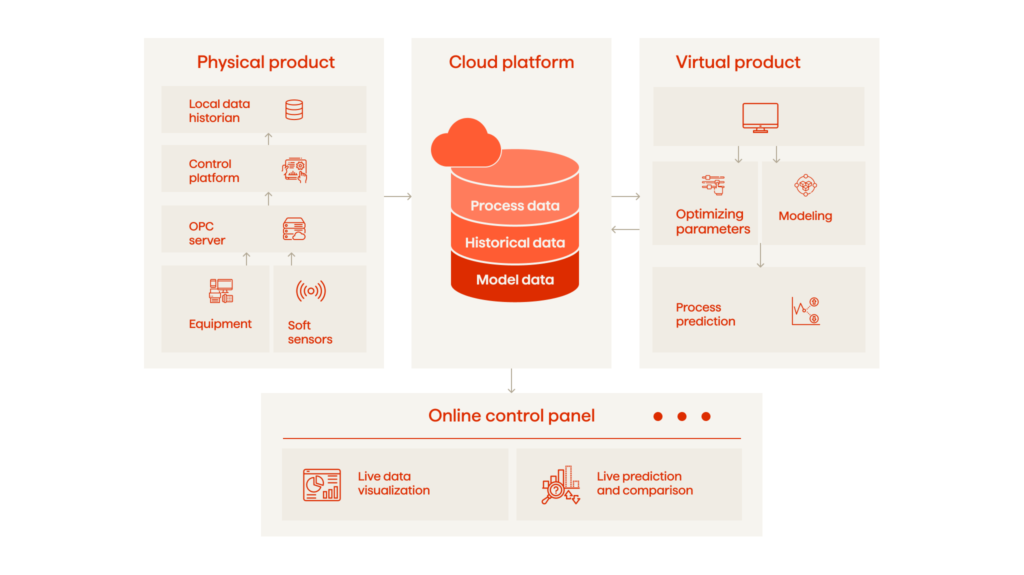
- Physical component. The physical segment of a DT includes all the tangible elements within the manufacturing environment. This encompasses sensors and network hardware, such as workstations and routers. These devices are responsible for gathering real-time operational data, essential for creating an accurate digital representation of the manufacturing process.
- Virtual component. A virtual component of a DT is a dynamically updated model that represents physical objects or processes. It involves the use of advanced technologies to create a digital representation of the physical world, such as virtual reality (VR), augmented reality (AR), 3D graphics, and data modeling. The virtual model is a responsive entity that evolves based on the continuous stream of data it receives. It serves multiple purposes, including performance monitoring, scenario testing, issue prediction, and optimization.
- Integrated data management system. At the heart of a digital twin is an integrated data management system, which manages the flow of information. It includes databases that store historical and current data, data transfer protocols that outline how data is communicated, and operational and model data that provide the necessary inputs for the virtual model. This system allows the DT to function as a cohesive unit, providing insights and enabling decision-making for process optimization.
- Data communication. It is the critical link that connects the physical and virtual components. Data communication involves the automatic exchange of data between them, facilitated by the integrated data management system. This system ensures that the virtual model is maintained with up-to-date information reflecting the real-time status of the physical operations.
These components form the core of a DT, enabling it to function as a dynamic tool that provides valuable insights into the performance, maintenance, and life cycle of the physical entity it represents.
The Role Of DTs in Biopharmaceutical Manufacturing
Digital twins can revolutionize biopharmaceutical manufacturing by simulating and optimizing processes, ensuring quality, and predicting maintenance needs, as you can see below:
- Process optimization. Digital twins offer a comprehensive simulation of biopharmaceutical processes, providing a virtual environment where every aspect of the manufacturing process can be analyzed and optimized. They enable the modeling of complex biological processes and the interaction between various components within a production line. By utilizing real-time and historical data, digital twins can simulate different operational scenarios to identify the most efficient pathways for drug production. This includes optimizing the flow of materials, energy consumption, and labor allocation. The goal is to create a lean manufacturing process that minimizes waste and maximizes output.
- Quality control. Digital twins play a crucial role in maintaining quality control. They provide a detailed virtual model that reflects the physical manufacturing process, allowing for the continuous monitoring of critical quality attributes and process parameters. By comparing real data against established benchmarks, digital twins can detect anomalies that may affect product quality. This proactive approach ensures that any deviations are corrected promptly, safeguarding the consistency and safety of the biopharmaceutical products. Moreover, digital twins facilitate the implementation of Quality by Design (QbD) principles, an approach that involves designing and developing pharmaceutical formulations and manufacturing processes to ensure predefined product quality. It’s about building quality into a product from the beginning, with a thorough understanding of how various factors affect the final product.
- Predictive maintenance. DTs are able to forecast equipment malfunctions and process deviations by analyzing data trends and machine learning algorithms. The predictive insight allows the scheduling of maintenance activities before failure occurs, thus avoiding unplanned downtime and production losses. DTs can also simulate the impact of maintenance activities on the overall production schedule, ensuring that interventions are timed to minimize disruption. The result is a more reliable manufacturing process with increased uptime and prolonged equipment life.
As we can see, DTs enhance the precision, efficiency, and reliability of manufacturing operations. Their ability to provide deep insights into the manufacturing process, from the molecular level to the entire production ecosystem, makes them an invaluable tool for companies looking to realize the full potential of Industry 4.0.
Learn about how Avenga automated internal budget processes for a leading biopharma enterprise, enabling better resource management and optimization of financial operations. Success story
Benefits Of DTs
Now that you know the common uses, let’s examine how digital twins enhance efficiency, help ensure compliance, and reduce costs, ultimately leading to a more sustainable production process in biopharma. The three primary benefits of DTs are:
- Efficiency. DTs act as a dynamic model, mirroring every facet of the production process. Virtual modeling enables manufacturers to conduct thorough analyses and what-if scenarios without disrupting actual production. The result is a streamlined process where adjustments and improvements can be made swiftly and confidently, leading to a significant reduction in cycle times and an increase in overall throughput.
- Enhanced compliance. Adhering to regulations is not only mandatory but also helps organizations stay competitive in the highly regulated biopharmaceutical industry. Thanks to their ability to comprehensively surveil and document the manufacturing process, digital twins are an ally in compliance. From the first design to the last product delivery, they ensure that every stage complies with strict regulatory requirements. Digital twins help preventatively detect and address compliance concerns by invigorating processes and controls, which enhances quality control in biopharma manufacturing itself.
- Reduced costs. Digital twins’ predictive capabilities and process optimization enable cost reduction. They provide foresight into potential system inefficiencies and failures, allowing for preemptive action to avoid costly downtime and extend the lifespan of equipment. By optimizing the use of materials and energy, digital twins help in creating a more sustainable process of manufacturing. Furthermore, they assist in reducing waste by ensuring that materials are used precisely and efficiently, leading to a decrease in overproduction.
To sum up, the DT technology brings measurable benefits to biopharma manufacturing. It streamlines production, helps to adhere to regulatory standards, and reduces operational costs.
DT Use Case In Biopharma Manufacturing
There are two recognized methods for producing biopharmaceuticals: batch processing and continuous manufacturing (CM). The conventional batch approach is a method where the production of biological drugs is carried out in set, controlled quantities or “batches.” Each batch goes through a series of steps, such as preparation, modification, and purification, before moving on to the next stage. Unlike batch processing, which divides the synthesis of active pharmaceutical ingredients into distinct parts, CM occurs in one continuous step. Similar to an assembly line, this continuous flow requires charging materials and discharging products.
The transition from traditional batch processing to CM in the biopharmaceutical industry has been evolving for several years, but a significant push came with the FDA’s support for modernizing drug production. This transition signifies a move towards efficiency and integration. An advanced dynamic-control system that can interact with products and equipment in real time is necessary due to the complex nature of CM. Such a system ensures accurate dosing of starting ingredients, such as culture media, synchronizing their addition with the process’s rate of consumption.
Although CM has long been used in the small-molecule drug manufacturing process, its uptake in the biopharmaceutical industry’s downstream activities has been modest. Here’s where DTs become useful. By suggesting or automatically modifying pump speeds, DTs can improve efficiency in diafiltration and other downstream processes. This optimization could simplify production by drastically lowering the number of cycles needed.
DTs have the ability to quickly convert the high dimensionality, format complexity, and diversity of measurements in biopharmaceutical activities to findings that can be put into practice. For example, taught by appropriate specialists in the field, DTs guarantee to continuously create all batches in predicted circumstances without human supervision. When the industry uses AI-enabled DTs, bioprocess comprehension, development, and control are improving dramatically.
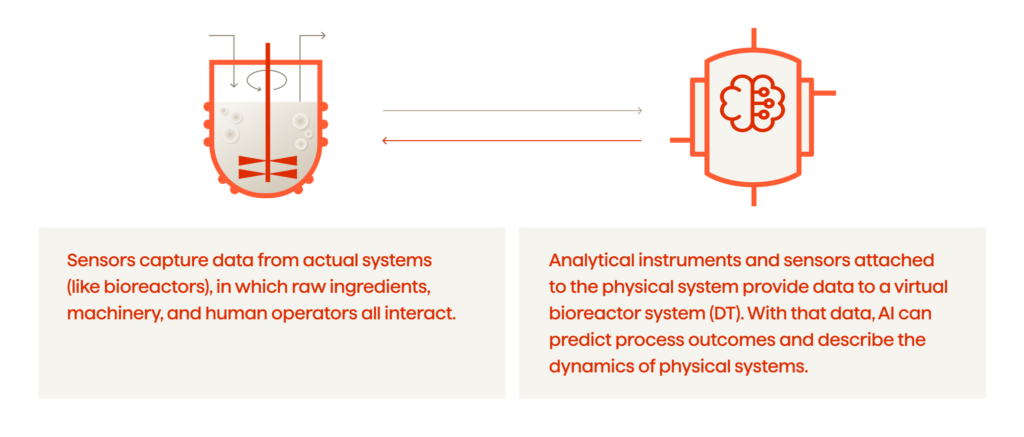
Challenges And Considerations
Digital twins promise significant advancements in biopharmaceutical manufacturing, yet their adoption is not without challenges. The following issues should be addressed to fully capitalize on the technology:
- Financial challenges. High initial costs can be a significant barrier for small to medium-sized enterprises. To ensure that staff members can use the technology effectively, the complexity of the system necessitates the creation of new operational procedures as well as a sizable investment in training and development. Additionally, DTs demand ongoing support and updates, adding to the long-term operational costs.
- Data security and privacy. The extensive use of data analytics in DTs raises concerns about data security and privacy. Biopharmaceutical manufacturers must ensure compliance with global data protection regulations, such as GDPR, and implement modern cybersecurity measures. The integrity of sensitive data, such as proprietary manufacturing processes, is critical. A breach could have far-reaching consequences, not just legally and financially but also in terms of consumer trust and brand reputation.
- Integration with existing systems. Integrating DTs with existing systems is a multifaceted challenge. It requires a seamless melding of the digital twin platform with legacy hardware and software, which may not have been designed for such integration. The process involves not only technical adjustments but also a rethinking of existing workflows and data management practices. Unlocking the full potential of digital twins requires effective integration in order to provide a unified perspective of the production process and improved decision-making.
Integrating digital twin technology in biomanufacturing is a complex procedure that requires meticulous planning and execution. Addressing the challenges of implementation, data security, and system integration is essential in order to achieve a new level of quality in drug production.
Prospects For DTs
Now let’s talk about the innovations that are expected to drive the evolution of DTs in the biopharmaceutical manufacturing process, making it even more efficient and compliant with regulatory standards:
- Process analytical technology (PAT) integration. DTs are expected to be deeply integrated with advanced PAT, allowing for real-time monitoring and control of the manufacturing. This integration will enable a more responsive and adaptable manufacturing environment, where adjustments can be made on the fly to ensure optimal performance.
- Enhanced process modeling. DTs will benefit from more sophisticated process modeling techniques that can accurately simulate complex biopharmaceutical processes. These models will help in understanding intricate relationships between various process parameters and the quality of the product.
- Data integration and virtual plant communication. The next generation of DTs will likely feature improved data integration capabilities, facilitating communication between the virtual and physical plants. This will allow for better synchronization between the digital model and the actual process of manufacturing.
- AI-driven process optimization. Artificial intelligence will play a crucial role in analyzing data from DTs to optimize biopharmaceutical manufacturing. AI algorithms can identify patterns and insights that human operators might miss, leading to more cost-effective production strategies.
- Real-time monitoring and analysis. DTs will advance to provide more detailed and comprehensive real-time process monitoring and analysis, enabling manufacturers to detect and address issues immediately, reducing downtime, and improving the overall quality of the manufacturing.
- Quality by Design integration. DTs will be instrumental in integrating QbD principles into biopharmaceutical manufacturing. By simulating the entire manufacturing, DTs can ensure that quality is built into the product from the earliest stages of development.
Apart from embracing these innovations, several recommendations are generally proposed to realize the potential of digital twins by biopharma companies:
- Strategic planning and investment. Organizations should develop a strategic plan that outlines the role of DTs in their long-term vision. Such a plan includes allocating appropriate budgets for technology acquisition, infrastructure upgrades, and workforce training. Investments should focus on acquiring high-quality sensors and advanced analytics platforms that can supply precise data to DTs.
- Data management and governance. A robust data management framework is essential for DTs to function effectively. Companies should establish clear data governance policies to ensure data quality, integrity, and security. This includes standardizing data formats, ensuring proper data storage, and implementing strict assessment control.
- Workforce development. The successful implementation of DTs requires a workforce with interdisciplinary skills, including data science, system engineering, and domain-specific knowledge. Companies should invest in training programs to upskill existing employees and consider hiring new specialists.
- Collaboration with regulatory bodies. As DTs are relatively new in the biopharmaceutical sector, working closely with regulatory bodies is essential. Companies should engage in a dialogue with regulators to make sure that the use of DTs aligns with compliance requirements and to help shape future guidelines that support innovation.
- Continuous improvement. DTs cannot be seen as a one-time investment but only as part of a continuous improvement process. Organizations should encourage a culture of innovation where feedback from DTs drives process enhancements and product development.
- Scalability. Scalability is an important consideration when designing DTs, as it allows for future growth and manufacturing modifications. This involves the use of readily upgradeable and reconfigurable hardware and software components that are modular in design.
- Integration with other Industry 4.0 technologies. DTs should be integrated with other Industry 4.0 technologies, such as AI, IoT, and robotics, to create a cohesive and intelligent manufacturing ecosystem. This can lead to greater automation and enhanced operational productivity.
- Customization for specific needs. DTs have to be tailored to the unique requirements of the biopharmaceutical production industry. This entails customizing the DTs to the distinct qualities of the created goods and the particular difficulties the business faces.
By adhering to these guidelines and adopting the aforementioned innovation tactics, biopharmaceutical enterprises can optimize the advantages of digital twins and become competitive in the marketplace.
Conclusion
DTs are expected to be essential in helping producers fulfill the demands of a dynamic and competitive market as the biopharmaceutical industry continues to innovate. With the use of real-time data, predictive analytics, and complex simulations, digital twins provide a strategic advantage by facilitating more informed decision-making and prompt responses to operational demands.
As a company that prioritizes operational efficiency and technological innovation, Avenga is ideally positioned to work with businesses looking to explore the full potential of digital twins.
Get in contact with us right now to enhance the quality of your products and manufacturing efficiency through the clever use of DT technology.