Artificial Intelligence and Machine Learning in Pharmacovigilance

January 29, 2026 17 min read
Find out about the insights pointing toward the current and prospective value of Artificial Intelligence and Machine Learning in Pharmacovigilance
Artificial Intelligence (AI) and Machine Learning (ML) are the most recognized technologies right now. While having a range of applications, they are taking pharmacovigilance to the next level.
Here, we will explore the phenomenon of Pharmacovigilance (PV). Through use cases and prospects, we see how Artificial Intelligence and Machine Learning in pharmacovigilance are reinventing the practice and creating more value. In turn, this means greater patient outcomes, better medicine, and new avenues for revenue.
The Value of Artificial Intelligence Systems and Machine Learning in Healthcare
Gartner indicates worldwide AI software revenue to reach $62.5 billion in 2022. It is a 21.3 percent increase compared to 2021. The numbers show that companies and industries are implementing AI on a major scale.
Why do businesses use AI and ML in particular?
In short, AI is the technology of replicating human intelligence. It can help tackle complex and high-dimensional data. In turn, ML employs traditional and deep learning capabilities. It allows for the making of accurate predictions and the painting of the precise classification of data points. Working in tandem, AI and ML are perfect for making sense out of an immense amount of data.
Companies can apply AI and ML to a wide range of industries and markets. With the application of them picking up speed, AI and ML implementations are visible in the healthcare industry as well. Research by USM Systems points out that 50 percent of global healthcare companies plan to implement AI by 2025.
Notably, the pharmaceutical industry shows promising outcomes and opportunities for AI and ML as well.
AI and ML in Pharmaceuticals
The process of drug discovery entails working with massive data input. This is when AI and ML step in. Here is an illustration of how pharma is ready for further AI implementation.
Statista shows 59 startups using AI to generate novel candidates in drug discovery. While drug discovery remains at the top of the list, there are other notable areas where AI and ML are used (see Fig. 1).
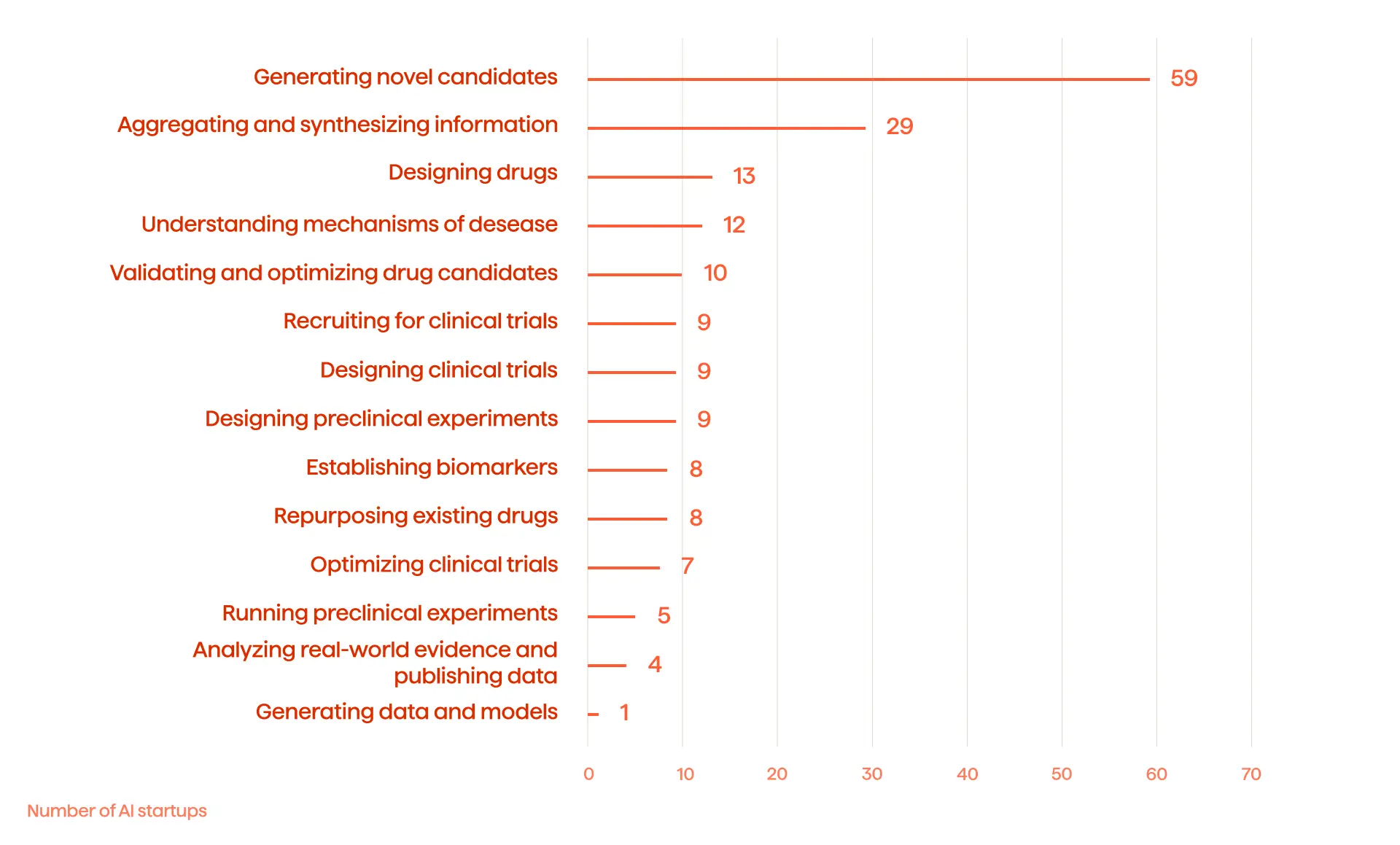
Why drug discovery?
Its prospects for growth and profit are key reasons. This research illustrates that the global AI, in the drug discovery market, will reach $1,434 billion by 2024, compared to $259 million in 2019. There is 40.8% projected annual growth. Here is more information on digitalization in drug discovery.
Along with drug discovery, there are a range of areas where companies implement AI and ML:
- Drug Screening
- Designing Drug Molecules
- Advancing Pharma Product Development
- Pharma Manufacturing
- Quality Control and Assurance
- Clinical Trial Design
- Market Positioning
- Market Predicting and Analysis
- Product Cost Estimation
As the research published by one of the most notable medical journals suggests, AI and ML in healthcare will change the approach to decision-making while making medicine more personalized to the needs of every patient.
Now, it is time to narrow down the focus and explore what PV is and how it changes pharma at this moment.
What is Pharmacovigilance?
In a nutshell, pharmacovigilance (PV) is the practice of including both science and the activities related to detection, understanding, and the prevention of various drug-related safety issues. Assessment of adverse drug reactions (ADRs) is PV’s primary task. In fact, there is data pointing out ADRs responsibility for nearly 7 percent of all hospital admissions in the United States alone. That is why PV is so vital.
Currently, the global PV market is expanding fast. The experts from Grand View Research indicate the U.S. PV market is worth $6.97 billion as of 2022. The expansion is predicted to reach a compound annual growth rate of 10.5 percent from 2022 to 2030 (see Fig. 2)
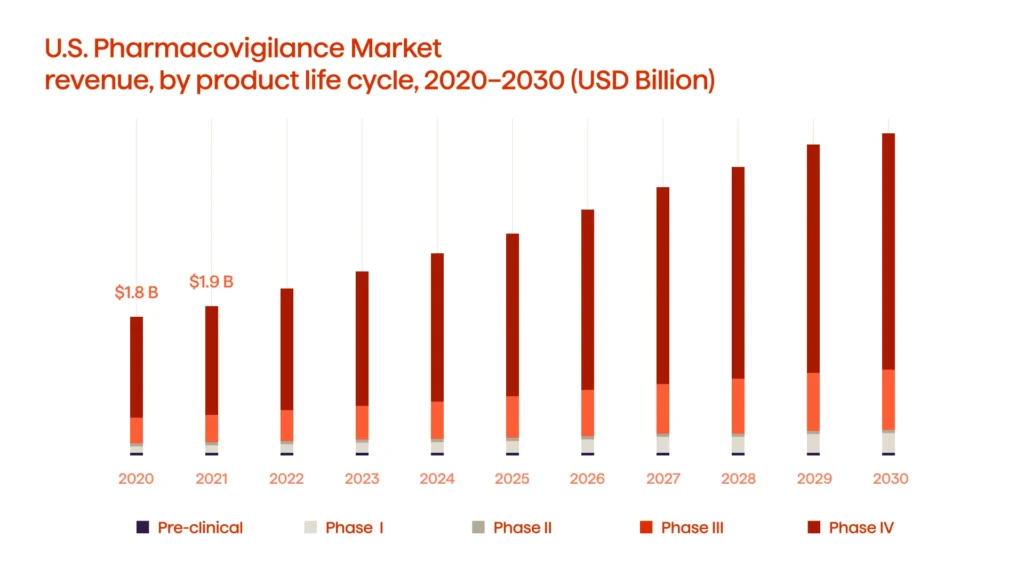
It’s prime time for companies to invest in PV. While the practice is developing, it also offers a range of application areas.
Areas of Application
Companies working on how drug-related safety is engaged is a matter of great importance. PV helps improve the process and ensures new drugs will be less harmful and more useful than the previous ones. Achieving this outcome relies on a range of areas that PV covers:
- Quantifying Drug-Related Harm to Patients. This aspect entails finding drug-related issues and identifying solution strategies to avoid potential drug harm. Quantifying risk versus benefit presents a credible way of assessing a drug’s effectiveness.
- Communicating Drug Risk. Population risk is this practice’s focus. PV helps test population risk and the incidence of ADR in the selected groups. The resulting knowledge provides clinicians with tools for communicating drug risks to individual patients.
- Heterogeneity of Patient Response. Working as detectives, experts in PV try to determine the heterogeneity of patient response to drug therapy. Their hard work will provide critical information to data clinicians as they choose the best drug therapy for a patient.
- Patient-Focused Risk Communication. PV explores new ways of designing patient-focused management solutions for ADRs. Comparing different ADR reports makes patient-specific risk information more accurate. In turn, it decreases the incidence of ADRs.
- Quality of ADR Data. The principal goal of PV is to improve drug safety. The quality of ADR data represents a major barrier to meeting such a goal. The future of pharmacovigilance depends on the ability to analyze massive amounts of information to boost the ADR’s data quality.
- Active Surveillance. Gathering ADR data relies upon evaluating patients’ real-time reactions to particular drug treatments. PV introduces various tools for active surveillance, which makes the process easier for clinicians, who in turn can offer valuable insights concerning patient-specific ADRs.
- New Drug Development and Drug Repurposing. New drug development and drug repurposing focus on making drugs safer. PV helps find the mechanistic basis of ADRs, which translates into greater drug efficacy and reduced toxicity.
These areas of application specifically focus on working a way around ADRs, which is another way PV helps make drugs safer. It also ensures clinicians have tools to help assign the drug therapy that follows the needs of individual patients. Yet, despite all the benefits and areas of application, there are limitations to traditional PV.
Challenges of Traditional Pharmacovigilance Solutions
For traditional PV, rising costs are a major challenge. The formula is simple: to improve patient outcomes, it is crucial to have high-quality ADR reports. Having such reports relies on data gathering techniques and the ability to analyze them. Companies and organizations need to ensure a massive investment into introducing such techniques on a major scale.
As a result, traditional PV costs more and more with the increasing stream of patient-specific ADR data. Following a recent Deloitte report, 90 percent of companies working with PV are looking for solutions to cut the costs of the approach.
The exponentially rising number of available data points is another challenge for traditional PV.
Several authors vocalized their concerns in the following publication, which is offered by the experts from the International Society of Pharmacovigilance (ISoP). There will be an exponential increase in ADR-related data inflow because of the growing global population. Unfortunately, methods used in traditional PV are not suitable to handle such an enormous amount of data.
In other words, traditional PV is expected to cost more while not processing all the upcoming data. While many look for alternatives, AI and ML seemingly offer solutions to the challenges traditional PV faces.
When AI and ML Meet the Pharmacovigilance Process
At this point, PV depends upon its ability to process data in a cost-efficient manner. Luckily, when AI and ML are integrated into this area they can do so, and much more. There is an array of potential solutions that AI and ML bring to the table:
- Cutting costs. Information from Deloitte’s report indicates that AI pharmacovigilance can potentially reduce the cost of the drug screening process by a staggering 80 percent.
- Better data analysis. Artificial Intelligence and Machine Learning in pharmacovigilance can help collect and analyze massive amounts of data, while eliminating human factors. Free-form text data is prevalent in pharma and healthcare in general, which makes AI and ML an even more efficient option.
- Automation. Integration of AI and ML brings a greater degree of automation in repetitive and routine tasks. This process will free up valuable resources which will help professionals focus on more value-adding objectives.
- Improved risk-benefit assessment. AI and ML can help improve the detection of potential drug-related events correlated to specific populations. They will boost the risk-benefit evaluation of new and existing drugs on the market.
- Extended outreach. Artificial Intelligence pharmacovigilance can work not only with healthcare resources, but the tools can tap into social media, news articles, and other public domain information. This will make ADR-related predictions more accurate, as well as offering real-world intelligence to improve personalized medicine.
These solutions are among the top benefits AI and ML bring into PV, especially when applied to pharma and healthcare in general. Statista points out that 60 percent of companies using AI expect the technology to help improve quality control in the pharma and healthcare industry. Besides, businesses can use AI in an assortment of additional ways (see Fig. 3).
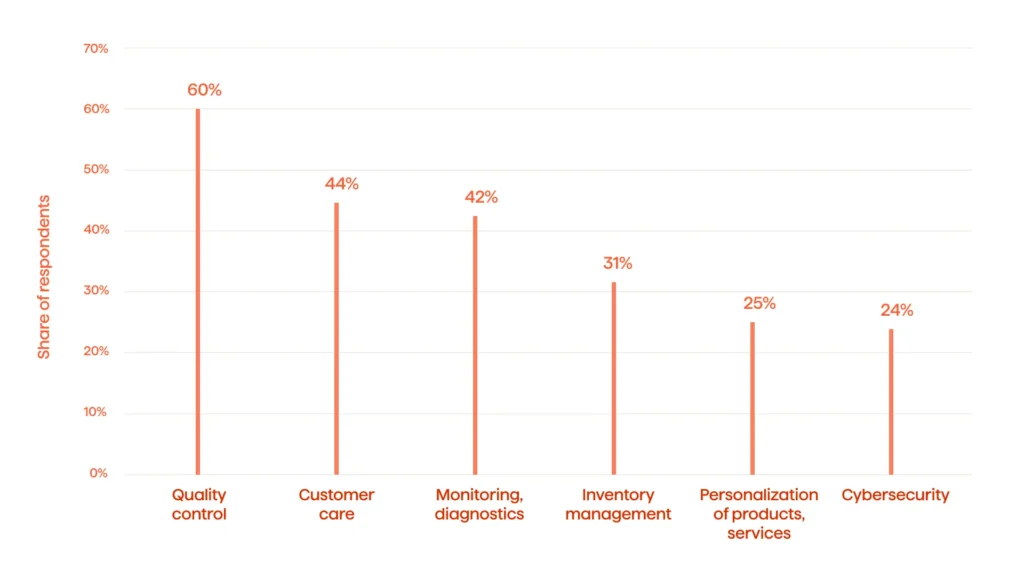
There is a bright future for AI and ML in pharma. Yet, to understand the practical applications of PV, current use cases should be examined.
Artificial Intelligence and Machine Learning Pharmacovigilance Use Cases
Use cases more clearly illustrate what AI and ML offer to PV. They are a practical illustration of how companies applied the technologies and what outcomes they received.
Predictive Analytics
The scholarly article published by Drug Discovery Today argues that AI helps to manage massive chunks of data while ML helps make predictions based on that analysis. Working in tandem, AI and ML improve the speed of the go-to-market time for new drugs. The typical drug design lifecycle takes about 10-15 years. With AI and ML, professionals use statistical models to get valuable insights from past, present, and future events, which drastically reduces the drug design lifecycle
SciBite uses the full potential of predictive analytics brought by AI and ML. Applying AI to its R&D model, the company reduced the time of new drugs going to the market. New York University reports an estimated 80 percent of clinical data as being unstructured. AI and ML are the tools that can work with such a massive information segment as a means to boost processes within the PV area.
Social Listening for Accurate Health and Drug-Related Information
Gathering data through social media can be a daunting task. But, with the right tools at hand, social media platforms can offer a great deal of important information. The article published after the Pacific Symposium on Biocomputing shows how analyzing five million posts with AI can grant significant insights into the effectiveness of antidepressants. In addition, the study highlights the importance of social listening in determining drug safety combinations and ADRs.
Researchers actually managed to discover new adverse effects of several existing drugs. They utilized AI to analyze peoples’ posts within the public domain and gained information that other health professionals missed due to outdated data analysis instruments.
Improved Automation of Regulatory and Safety Systems
Adherence to regulatory and safety standards is the bedrock of PV. Regulators use ADR reports and individual case safety reports (ICSRs) to design new safety standards. With Artificial Intelligence and Machine Learning in pharmacovigilance, regulatory bodies can obtain a clearer picture of drug safety. It means greater capabilities in protecting consumers from hazardous products.
The research published by the National Center for Biotechnology Information shows how Celgene’s Global Drug Safety and Risk Management (GDSRM) pioneered in utilizing AI across the PV value chain to develop rigorous drug safety standards. Moreover, the evidence suggests working on drug safety is a prospective realm showing great promise. In 2019, the PV and drug safety software market was worth $160.67 million. By 2027, it is expected to reach $292.97 million (see Fig. 4).
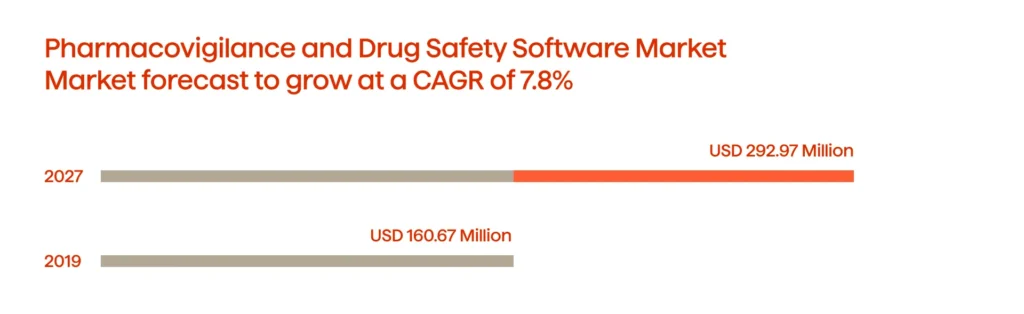
Developing proper drug safety regulations and standards improves patient outcomes and businesses’ profitability.
Automated Case Processing
In PV, 40 to 85 percent of costs are allocated to case processing. As a result, the primary objective of leaders in the market is to drive costs out of case processing. This can be achieved through automation. A report by Deloitte shows how the integration of AI-based automation in case processing saves up to 30 percent of costs per ICSR.
Supporting Clinical Trial Efficiency, Interoperability, and Effectiveness
AI and ML in PV offer major benefits in automating patient safety data and optimizing clinical trial effectiveness. The United States National Institute of Health offers data encapsulating the broad impact of AI on the elements mentioned earlier.
In short, the case shows how AI-based technologies like signal detection, risk conceptualization, and tracking, coupled with ML algorithms, provide PV professionals with the leverage needed to achieve clinical trial efficiency, interoperability, and effectiveness within an economically feasible manner. Notably, some companies are already using AI to develop clinical trial software.
These use cases portray AI’s value, and how ML now creates PV. What is more, it’s time to dive into some prospective areas expected to bring even more value in the future.
Future Opportunities for Artificial Intelligence and Machine Learning in Pharmacovigilance
The gist of all this is that AI and ML create value through data collection, analysis, and prediction algorithms. Looking into the future, we can see several areas of PV that will benefit from AI and ML implementation.
Smarter Individual Case Safety Report (ICSR) Collection
Proper collection of ICSR reports represents a major issue in PV. Their analysis is an even greater challenge. The study published in a journal, Clinical Pharmacology & Therapeutics, suggests that the World Health Organization (WHO) database holds over 20 million ICSRs, which could grant vital insights on drug safety and ADRs. With the introduction of AI and ML into the ICSR collection process, the entire system becomes smarter.
The professionals predict that by 2030, ICSR reporting will be way more advanced than it is now. AI-based tools like Natural Language Processing (NLP) can analyze massive amounts of unstructured text within ICSRs, giving rise to AI-augmented ICSR management.
Cloud-Based Reporting
AI pharmacovigilance goes hand-in-hand with cloud-based computing. The experts anticipate data being collected and analyzed through available cloud technologies. It is expected that coupling cloud-based computing with AI and ML will improve PV’s cost-efficiency, scalability, and simplicity.
Social Media and Digital Health
Social media is and will continue to play an integral role in healthcare. The research offered by PWC shows 90 percent of people between the ages of 18 and 24 say that they would trust medical information presented via social media networks. Besides, the research published in the journal BMC suggests 87.9 percent of healthcare professionals use social media for personal and work matters. It all comes down to this point – people use social media extensively and healthcare professionals can employ this phenomenon for bettering public health.
As to PV, the authors of the book The Era of Artificial Intelligence, Machine Learning, and Data Science in the Pharmaceutical Industry collectively point out that social media offers untapped potential for data extraction and analysis. The analysis that particularly works in social media status updates can lead to a more accurate classification of the ICSR. In a nutshell, social media expands the outreach of PV experts, and AI and ML help translate it into safer drug production.
Personalized Medicine
Personalized medicine revolves around identifying a person’s biological, physical, physiological, and genetic markers in order to tailor individually optimized therapies. With Artificial Intelligence in pharmacovigilance, healthcare professionals can analyze thousands of markers and make much more accurate predictions on how specific drugs will affect particular individuals. Inevitably, drug therapies will become increasingly personalized, thus decreasing ADRs and improving drug efficiency.
Nanomedicine and Drug Delivery
Nanomedicine is not a realm of science-fiction, but instead is a reality now. The study published in the scholarly journal Drug Discovery Today shows how trailblazers use a combination of nanotechnology and medicine to diagnose, treat, and monitor a range of complex conditions. Experts work with HIV, cancer, malaria, and asthma. While nanomedicine is still in its inception, there are breakthroughs in the aspect of nanoparticle-modified drug delivery.
Recently published research from a scholarly journal indicates scientists and engineers are working on creating implantable nanorobots which are being developed for the better delivery of drugs. AI tools like NNs (neural networks), fuzzy logic, and integrations can mitigate the entire process. Here, you can learn more about nanorobots and drug delivery.
The introduction of AI and ML into PV grasps only a part of the entirety of the pharmaceutical trends that are earning value from digitalization. While there are many prospective instruments on the way, professionals need to consider the array of challenges associated with the AI and ML integration into pharma and healthcare.
Barriers of Artificial Intelligence and Machine Learning in Pharmacovigilance within Healthcare
AI and ML work with massive amounts of data, which entails collection, analysis, storage, and synthesis. Naturally, when there is data, some prerequisites need to be met. These include the following:
- Data Governance. Work with complex and unstructured data should be based upon pre-designed methods. While AI and ML can handle the data, people still need to properly program the algorithm.
- Data Science Professionals. Integrating AI and ML requires data science professionals to be involved. It means pharmaceutical and healthcare companies need to reserve space and resources for hiring these new types of experts, the ones familiar with AI and ML.
- Data Protection. People working with patient data must meticulously follow data security standards and guidelines. Naturally, further integration of AI and ML requires redesigning such measures to make them more up-to-date. But also, the systems working with sensitive data must entail high-grade encryption along with being HIPAA compliant.
- Intellectual Property right. AI and ML bring forward the issue of intellectual property rights. As more businesses switch to AI- and ML-driven approaches, the existing case law will have to change in order to ensure AI-driven biotech and pharmatech inventions will not slip through the gaps of existing intellectual property law.
All in All
PV takes on the vital task of ensuring drug safety and improving patient outcomes. AI and ML prove to be of massive help when handling this objective. There is a very bright future for AI and ML in PV. Artificial Intelligence and Machine Learning in pharmacovigilance can create exponential value on many fronts.
In business terms, it means that investing in AI and ML along with data science, in general, is prospective. And, companies get a chance to make medicine safer while gaining profit along the way.
If you have any additional questions, feel free to contact us.